Parler
Parler Gab
Gab
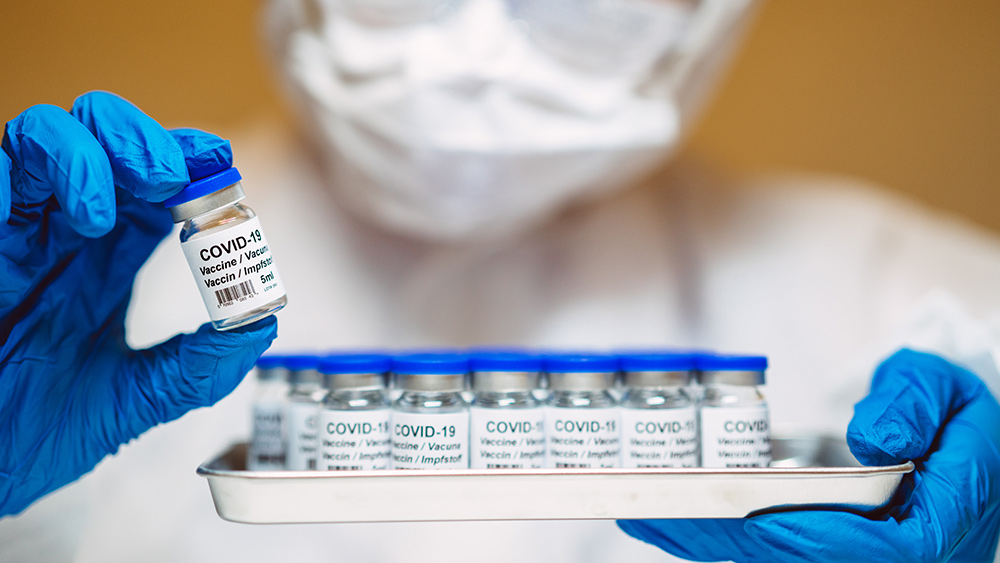
- HHS Secretary Robert F. Kennedy Jr. has directed the FDA to revise its safety regulations, removing the provision that allows companies to self-certify the safety of food ingredients under the "substances generally recognized as safe" (GRAS) rule.
- The proposed changes mandate that companies submit detailed safety data and intended use notifications to the FDA, ensuring greater transparency and accountability in the food industry. The FDA will maintain a public inventory of all notices and supporting data.
- Kennedy emphasized that the move aims to close a loophole that has allowed potentially unsafe ingredients into the food supply without FDA notification. The directive seeks to enhance consumer trust and improve public health by ensuring food ingredients are thoroughly vetted.
- The directive has been welcomed by some food industry partners, such as the Consumer Brands Association, which represents major companies like Coca-Cola and WK Kellogg. However, other major companies like PepsiCo and General Mills have yet to comment.
- The directive aligns with recent FDA initiatives, such as front-of-package nutrition labeling and restructuring its food division, signaling a more proactive approach to food safety regulation and oversight. Acting FDA Commissioner Sara Brenner affirmed its commitment to safeguarding the food supply.
Most food industry partners welcome RFK Jr.'s new FDA directive
The announcement has been welcomed by some food industry partners. For instance, the Consumer Brands Association, which represents companies such as Coca-Cola and WK Kellogg, said that they "look forward to continued engagement with the secretary and the qualified experts within HHS to support public health, build consumer trust and promote consumer choice." Meanwhile, major companies such as PepsiCo, General Mills, Kraft Heinz, Hershey, Mondelez and Kellanova have yet to comment on the directive. It also aligns with recent efforts by the FDA to bolster oversight of the food supply. Earlier this year, the agency proposed requiring food companies to display nutrition labels on the front of packaging, a measure aimed at helping consumers make healthier choices. The FDA has also been restructuring its food division under former Commissioner Robert Califf to enhance oversight of food and agricultural products. Kennedy's directive builds on these efforts, signaling a more proactive approach to food safety regulation. "The FDA is committed to further safeguarding the food supply by ensuring the appropriate review of ingredients and substances that come into contact with food," FDA Acting Commissioner Sara Brenner posted on X, formerly known as Twitter, shortly after the announcement. Watch this video about RFK Jr. promising to address the existential threat of chronic disease. This video is from the JD Rucker channel on Brighteon.com.More related stories:
RFK Jr. halts oral COVID-19 vaccine trials, prioritizing safety over speed.
RFK Jr. reverses course, orders HHS staff to comply with Musk's controversial email demand.
Sources include: TheGuardian.com USNews.com HHS.gov Brighteon.comExcitotoxins: The hidden food additives that could harm your baby’s brain
By Olivia Cook // Share
Five herbs and spices to turn ordinary meals into ‘supermeals’
By News Editors // Share
Standing up for health: How less time sitting lowers blood pressure in seniors
By Lance D Johnson // Share
Lemon verbena: A fragrant powerhouse of wellness
By Ava Grace // Share
Biden-appointed judge blocks Trump’s transgender military ban
By Cassie B. // Share
Tesla owners targeted in alleged doxxing campaign as anti-Musk sentiment escalates
By Finn Heartley // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share