Parler
Parler Gab
Gab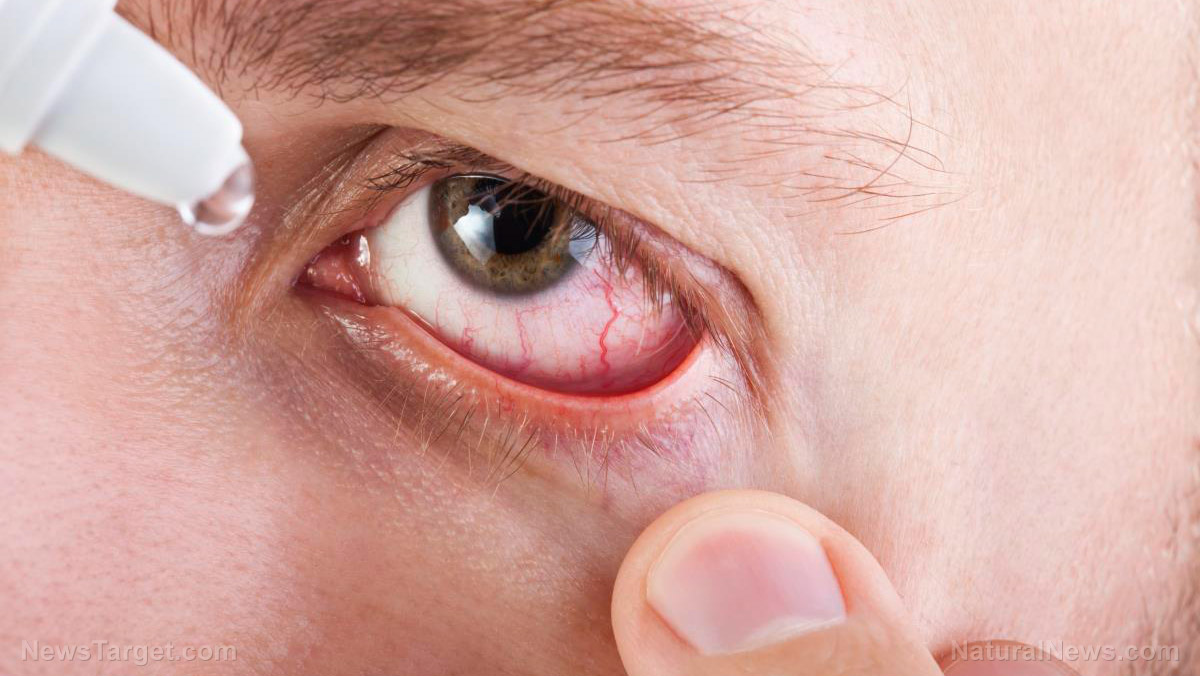
Eye products infected with drug-resistant bacterial strain
Concurrent with the voluntary recall notices, the FDA and the Centers for Disease Control and Prevention (CDC) cautioned consumers and medical providers against purchasing or using contaminated eye products. Back in February, the two agencies warned against using artificial tears made by the New York-based Delsam Pharma and the New Jersey-based EzriCare over potential contamination. They also extended this warning to Delsam's Artificial Eye Ointment. "Using contaminated artificial tears increases the risk of eye infections that could result in blindness or death. Patients who have signs or symptoms of an eye infection should talk to their health care provider or seek medical care immediately," the FDA said. According to the regulator, it also recommended that the ointment be recalled – which Delsam agreed to. A March 1 health alert from the CDC said about 61 people suffered were infected with a drug-resistant strain of the Pseudomonas aeruginosa bacteria across about a dozen U.S. states. The infections claimed the life of one person and caused loss of vision in at least eight. The majority of patients told the public health agency that they used artificial tears, noting more than 10 different brands. Lab tests performed by the CDC detected P. aeruginosa in some EzriCare bottles from several lots. "These specimens were collected in both outpatient and inpatient healthcare settings," the agency noted. Commonly found in soil and water, P. aeruginosa poses the most risk to people using ventilators or catheters, or among those with wounds from burns or surgery. (Related: FDA announces recalls for powdered milk products for fear of salmonella contamination.) EzriCare defended itself in a statement, emphasizing that it had "no role in the formulation" or manufacturing of the eye drops. It added: "We are not aware of any testing that definitively links the P. aeruginosa outbreak to EzriCare artificial tears." "Nonetheless, we immediately took action to stop any further distribution or sale of [the contaminated product]. To the greatest extent possible, we have been contacting customers to advise them against the continued use of the product." Visit FDA.news for more stories about product recalls. Watch this video about the FDA recalling two million Wuhan coronavirus (COVID-19) test kits over false positives. This video is from The Sword & Shield channel on Brighteon.com.More related stories:
CDC: Listeria outbreak linked to deli meat and cheese kills 1, infects 16. Over 2M at-home COVID-19 test kits recalled due to false positive results. FDA recalls frozen oysters in 13 states over fears of sapovirus contamination. FDA probing cases of FOOD POISONING from Lucky Charms breakfast cereal. Popular sunscreens recalled over cancer-causing ingredient; FDA investigating. Sources include: TheEpochTimes.com FDA.gov 1 FDA.gov 2 Brighteon.comHealth and nutrition: Can you eat processed foods while following a diet to reduce cancer risk?
By Rose Lidell // Share
‘Follow the money’: CBS wakes up to Wuhan lab’s ‘high-risk research’
By News Editors // Share
UK national security compromised by munitions shortage after sending aid to Ukraine
By Cassie B. // Share
Human rights activist warns: Pending WHO treaties to usher in TYRANNY in America
By Belle Carter // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share