Parler
Parler Gab
Gab
Pfizer slapped with huge fines for off-label promotion of Neurontin
For several years, both before and after the release of the New York Times investigation, Pfizer has been forced to pay steep penalties for its illegal off-label promotion of Neurontin. In 2004, four years before the investigation, Pfizer agreed to plead guilty to two felonies and pay a fine of $430 million to settle charges that it fraudulently promoted Neurontin for use in dealing with other disorders. In the settlement, now-defunct Pfizer subsidiary Warner-Lambert took all the blame for aggressively marketing the seizure drug for unrelated conditions, including bipolar disorder, general pain, migraine headaches and drug and alcohol addiction withdrawal. The settlement includes a criminal fine of $240 million, which at the time was the second-largest fine ever imposed on a Big Pharma company for healthcare fraud, and $152 million to pay back how much Medicare and Medicaid programs spent acquiring Neurontin. In 2010, a jury in a federal court in Boston once again found Pfizer guilty of marketing fraud due to Neurontin and told the company to pay $142.1 million in damages. The jury, which deliberated for two days on the case, found that Pfizer engaged in a racketeering conspiracy over a 10-year period and violated the Racketeer Influenced and Corrupt Organizations Act (RICO) under California's Unfair Competition Law. The case was brought up by Kaiser Foundation Health Plan and Kaiser Foundation Hospitals, which both claimed that Pfizer illegally promoted Neurontin for unapproved uses and that the Big Pharma company forced Kaiser to pay $90 million more than it should have for the seizure medication. In 2014, Pfizer was forced to pay steep fines twice in two different cases that were settled that same year. In April, 2014, Pfizer agreed to pay $190 million to settle a federal antitrust lawsuit claiming that the company attempted some illegal maneuvering to keep cheaper generic alternatives to Neurontin off the market. In June, 2014, Pfizer agreed to pay another $325 million to wrap up claims that another of its subsidiaries, Parke-Davis, promoted Neurontin for off-brand use without the approval of the Food and Drug Administration. Overall, Pfizer was fined around $1.087 billion from 2004 to 2014 for its illegal off-label promotion of Neurontin. Learn more about Pfizer and other Big Pharma companies at BigPharmaNews.com. Watch this clip from InfoWars featuring Israeli Prime Minister Benjamin Netanyahu admitting that Israelis were used as human guinea pigs for Pfizer's Wuhan coronavirus (COVID-19) vaccine. This video is from the InfoWars channel on Brighteon.com.More related stories:
NEVER FORGET: In 1994, Pfizer paid $20 million after LYING about defective heart valve that killed hundreds. Pfizer's criminal history is EXTENSIVE, Pfizer Files show. NEVER FORGET: Pfizer sued for $7B over illegal drug trials on kids. THREAD #PfizerFiles – the true history of Pfizer's repeated criminal behavior and destruction of human lives. Pfizer a "habitual offender" that "persistently" engages in illegal activity, study warns. Sources include: NYTimes.com MayoClinic.org SFGate.com Bloomberg.com FeircePharma.com Brighteon.comGovernment to blame sudden death epidemic on long COVID and climate change, says Ed Dowd
By Arsenio Toledo // Share
NEVER FORGET: Pfizer paid doctors $35M to “educate” other practitioners and market its drugs
By Belle Carter // Share
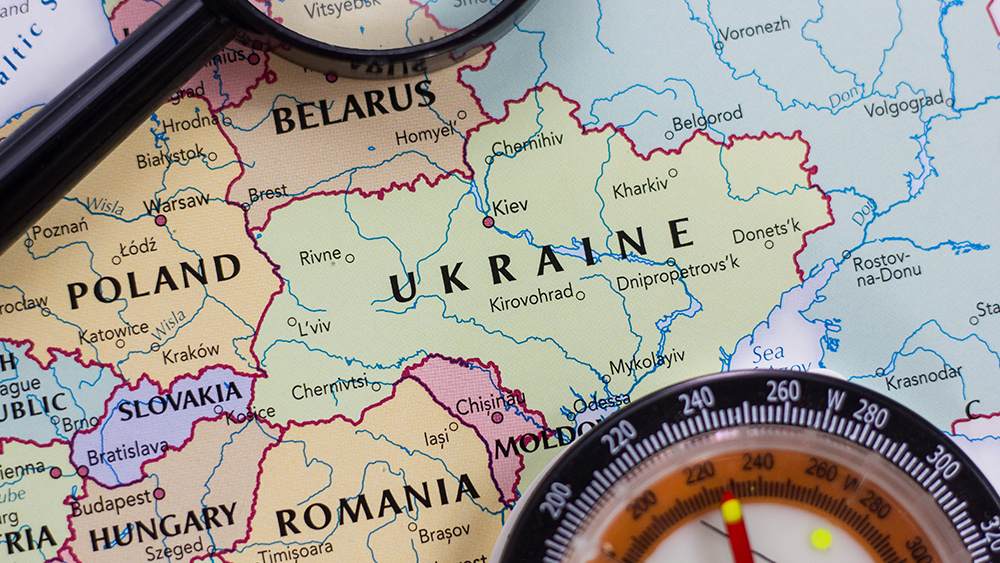
Ukrainian blogger calls for GENOCIDE of Russians: Wipe them off the face of the Earth
By Ramon Tomey // Share
Having a healthy, active lifestyle found to lower the risk of developing Alzheimer’s
By Olivia Cook // Share
Flame retardants in household items linked to chronic diseases
By Olivia Cook // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share